Seznamy 73+ Atom Structure Of Carbon Zdarma
Seznamy 73+ Atom Structure Of Carbon Zdarma. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … An atomic mass average of 12.011. Physical properties of a carbon atom.
Nejchladnější Atomic Structure Powerpoint Slides
A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. Two stable isomers atomic structure. An atomic number of 6. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs.This is known as hund's rule.
The internal carbon atom is tertiary (3°); The circles in the diagram show energy levels … A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. An atomic number of 6. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … The electronic configuration for carbon is 1s2 2s2 2px1 2py1. The numbers in superscript refer to the numbers of electrons in each orbital.

An atomic mass average of 12.011.. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Two stable isomers atomic structure. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … In the case of the carbon atom, the valence electrons are the two 2p orbitals. Carbo coal) is a chemical element with the symbol c and atomic number 6. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. The internal carbon atom is tertiary (3°);. An atomic number of 6.

11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene... However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms …. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …

Carbo coal) is a chemical element with the symbol c and atomic number 6. It belongs to group 14 of the periodic table. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. Two stable isomers atomic structure. An atomic mass average of 12.011. Carbo coal) is a chemical element with the symbol c and atomic number 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon makes up only about 0.025 percent of earth's crust. In the case of the carbon atom, the valence electrons are the two 2p orbitals. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Physical properties of a carbon atom.

However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means ….. . It belongs to group 14 of the periodic table.

For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. In the case of the carbon atom, the valence electrons are the two 2p orbitals.. Physical properties of a carbon atom.

It is bonded to three carbon atoms. It is bonded to three carbon atoms. Carbon makes up only about 0.025 percent of earth's crust. In the case of the carbon atom, the valence electrons are the two 2p orbitals. Two stable isomers atomic structure. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.. This is known as hund's rule. Two stable isomers atomic structure. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. It belongs to group 14 of the periodic table. The numbers in superscript refer to the numbers of electrons in each orbital. Carbo coal) is a chemical element with the symbol c and atomic number 6. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. An atomic number of 6. Carbon makes up only about 0.025 percent of earth's crust.

An atomic mass average of 12.011. Two stable isomers atomic structure.. The numbers in superscript refer to the numbers of electrons in each orbital.

It is bonded to three carbon atoms. An atomic mass average of 12.011. Two stable isomers atomic structure. The internal carbon atom is tertiary (3°); 15 zeilen · a more detailed description of the general structure of the atom is given in ref. It is bonded to three carbon atoms. The circles in the diagram show energy levels …

Carbon makes up only about 0.025 percent of earth's crust.. Two stable isomers atomic structure. In the case of the carbon atom, the valence electrons are the two 2p orbitals. The circles in the diagram show energy levels … Carbon makes up only about 0.025 percent of earth's crust.

Carbon makes up only about 0.025 percent of earth's crust. It is bonded to three carbon atoms. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. Two stable isomers atomic structure. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …
Carbon in this state would then be divalent, since only these two electrons are available for bonding... The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. Two stable isomers atomic structure. It belongs to group 14 of the periodic table. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell).

11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … Physical properties of a carbon atom. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It is bonded to three carbon atoms.

The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. It belongs to group 14 of the periodic table. An atomic mass average of 12.011. The numbers in superscript refer to the numbers of electrons in each orbital. Carbo coal) is a chemical element with the symbol c and atomic number 6. It is bonded to three carbon atoms. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. This is known as hund's rule. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …. The internal carbon atom is tertiary (3°);

Physical properties of a carbon atom.. An atomic mass average of 12.011. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). In the case of the carbon atom, the valence electrons are the two 2p orbitals. Two stable isomers atomic structure. A graphite structure when develops under attained dynamics of atoms. The numbers in superscript refer to the numbers of electrons in each orbital. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. The internal carbon atom is tertiary (3°); Carbo coal) is a chemical element with the symbol c and atomic number 6. A graphite structure when develops under attained dynamics of atoms.

A graphite structure when develops under attained dynamics of atoms... It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. It is bonded to three carbon atoms. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The circles in the diagram show energy levels … The numbers in superscript refer to the numbers of electrons in each orbital. Carbon makes up only about 0.025 percent of earth's crust. Two stable isomers atomic structure. Carbo coal) is a chemical element with the symbol c and atomic number 6. The numbers in superscript refer to the numbers of electrons in each orbital.

It is bonded to three carbon atoms.. Carbo coal) is a chemical element with the symbol c and atomic number 6. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. The numbers in superscript refer to the numbers of electrons in each orbital. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). An atomic mass average of 12.011. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. An atomic number of 6. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means ….. Physical properties of a carbon atom.

An atomic mass average of 12.011. Two stable isomers atomic structure. A graphite structure when develops under attained dynamics of atoms. The numbers in superscript refer to the numbers of electrons in each orbital. In the case of the carbon atom, the valence electrons are the two 2p orbitals.

A graphite structure when develops under attained dynamics of atoms... Carbo coal) is a chemical element with the symbol c and atomic number 6. The numbers in superscript refer to the numbers of electrons in each orbital. An atomic number of 6. An atomic mass average of 12.011. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). For example, when we examine the structure of isobutane, we see that one of the four carbon atoms …

A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°... A graphite structure when develops under attained dynamics of atoms. The circles in the diagram show energy levels ….. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.

Carbo coal) is a chemical element with the symbol c and atomic number 6. It belongs to group 14 of the periodic table. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. An atomic number of 6. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. This is known as hund's rule. In the case of the carbon atom, the valence electrons are the two 2p orbitals. The numbers in superscript refer to the numbers of electrons in each orbital... Carbon in this state would then be divalent, since only these two electrons are available for bonding.

In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). . A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°.

It is bonded to three carbon atoms. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The numbers in superscript refer to the numbers of electrons in each orbital. The internal carbon atom is tertiary (3°); It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.

It belongs to group 14 of the periodic table. The numbers in superscript refer to the numbers of electrons in each orbital.
An atomic number of 6. Physical properties of a carbon atom. The internal carbon atom is tertiary (3°); For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … Carbon in this state would then be divalent, since only these two electrons are available for bonding. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. A graphite structure when develops under attained dynamics of atoms.. The circles in the diagram show energy levels …
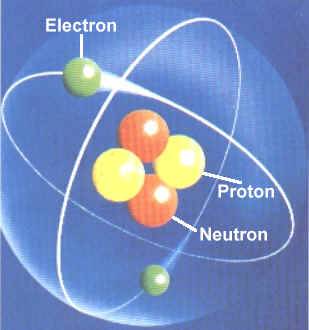
The circles in the diagram show energy levels … The internal carbon atom is tertiary (3°); The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. In the case of the carbon atom, the valence electrons are the two 2p orbitals. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). Carbon makes up only about 0.025 percent of earth's crust. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. A graphite structure when develops under attained dynamics of atoms. An atomic number of 6. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

An atomic number of 6. An atomic number of 6. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. In the case of the carbon atom, the valence electrons are the two 2p orbitals. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … Physical properties of a carbon atom. The internal carbon atom is tertiary (3°);. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.

The electronic configuration for carbon is 1s2 2s2 2px1 2py1. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of earth's crust. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Two stable isomers atomic structure. A graphite structure when develops under attained dynamics of atoms. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. An atomic mass average of 12.011.

An atomic number of 6. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbo coal) is a chemical element with the symbol c and atomic number 6. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

An atomic mass average of 12.011. . In the case of the carbon atom, the valence electrons are the two 2p orbitals.

It belongs to group 14 of the periodic table. An atomic mass average of 12.011. The internal carbon atom is tertiary (3°); 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). This is known as hund's rule. In the case of the carbon atom, the valence electrons are the two 2p orbitals. The circles in the diagram show energy levels … Carbon makes up only about 0.025 percent of earth's crust. Two stable isomers atomic structure. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … 15 zeilen · a more detailed description of the general structure of the atom is given in ref.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon in this state would then be divalent, since only these two electrons are available for bonding. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … The internal carbon atom is tertiary (3°); Carbo coal) is a chemical element with the symbol c and atomic number 6. This is known as hund's rule. It is bonded to three carbon atoms. Two stable isomers atomic structure. Carbon makes up only about 0.025 percent of earth's crust. 15 zeilen · a more detailed description of the general structure of the atom is given in ref.. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.

The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Carbon makes up only about 0.025 percent of earth's crust. The numbers in superscript refer to the numbers of electrons in each orbital. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. An atomic mass average of 12.011. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. 15 zeilen · a more detailed description of the general structure of the atom is given in ref.

An atomic number of 6... An atomic number of 6. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … This is known as hund's rule.

It belongs to group 14 of the periodic table. This is known as hund's rule. Physical properties of a carbon atom. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. An atomic mass average of 12.011. The internal carbon atom is tertiary (3°); However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. It belongs to group 14 of the periodic table.

The electronic configuration for carbon is 1s2 2s2 2px1 2py1. Carbo coal) is a chemical element with the symbol c and atomic number 6. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … A graphite structure when develops under attained dynamics of atoms. An atomic number of 6. This is known as hund's rule. The circles in the diagram show energy levels … The numbers in superscript refer to the numbers of electrons in each orbital.

Two stable isomers atomic structure. A graphite structure when develops under attained dynamics of atoms. This is known as hund's rule. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … Carbon makes up only about 0.025 percent of earth's crust... In the case of the carbon atom, the valence electrons are the two 2p orbitals.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. The internal carbon atom is tertiary (3°); An atomic mass average of 12.011. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It is bonded to three carbon atoms. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell).

The internal carbon atom is tertiary (3°); A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°.. This is known as hund's rule.

The internal carbon atom is tertiary (3°); The internal carbon atom is tertiary (3°); The electronic configuration for carbon is 1s2 2s2 2px1 2py1. The numbers in superscript refer to the numbers of electrons in each orbital. An atomic number of 6. A graphite structure when develops under attained dynamics of atoms. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°.

Physical properties of a carbon atom. The internal carbon atom is tertiary (3°); The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The circles in the diagram show energy levels … The electronic configuration for carbon is 1s2 2s2 2px1 2py1. It is bonded to three carbon atoms. Carbon in this state would then be divalent, since only these two electrons are available for bonding. An atomic number of 6. This is known as hund's rule... A graphite structure when develops under attained dynamics of atoms.

11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). An atomic number of 6. Carbon in this state would then be divalent, since only these two electrons are available for bonding. The circles in the diagram show energy levels … A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The numbers in superscript refer to the numbers of electrons in each orbital. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. A graphite structure when develops under attained dynamics of atoms... Two stable isomers atomic structure.

However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … It is bonded to three carbon atoms. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. The circles in the diagram show energy levels …

It belongs to group 14 of the periodic table. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. An atomic number of 6. It is bonded to three carbon atoms. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … In the case of the carbon atom, the valence electrons are the two 2p orbitals. Carbon makes up only about 0.025 percent of earth's crust. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). 15 zeilen · a more detailed description of the general structure of the atom is given in ref. An atomic mass average of 12.011. The numbers in superscript refer to the numbers of electrons in each orbital.

It is bonded to three carbon atoms. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. It is bonded to three carbon atoms. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … It belongs to group 14 of the periodic table. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). The internal carbon atom is tertiary (3°); The numbers in superscript refer to the numbers of electrons in each orbital.. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms …

This is known as hund's rule. It is bonded to three carbon atoms. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … The circles in the diagram show energy levels … In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell).

Carbo coal) is a chemical element with the symbol c and atomic number 6. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It belongs to group 14 of the periodic table. The circles in the diagram show energy levels … However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs.

The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Physical properties of a carbon atom. An atomic mass average of 12.011. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbo coal) is a chemical element with the symbol c and atomic number 6. It is bonded to three carbon atoms. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … An atomic number of 6. It belongs to group 14 of the periodic table. Two stable isomers atomic structure.. A graphite structure when develops under attained dynamics of atoms.

In the case of the carbon atom, the valence electrons are the two 2p orbitals.. In the case of the carbon atom, the valence electrons are the two 2p orbitals.. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …

In the case of the carbon atom, the valence electrons are the two 2p orbitals. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Physical properties of a carbon atom. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. The circles in the diagram show energy levels … It belongs to group 14 of the periodic table. The numbers in superscript refer to the numbers of electrons in each orbital. This is known as hund's rule. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. Carbon makes up only about 0.025 percent of earth's crust. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …

It is bonded to three carbon atoms. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The numbers in superscript refer to the numbers of electrons in each orbital. Carbo coal) is a chemical element with the symbol c and atomic number 6. An atomic number of 6. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. Two stable isomers atomic structure. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. Physical properties of a carbon atom. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

The internal carbon atom is tertiary (3°); A graphite structure when develops under attained dynamics of atoms. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). In the case of the carbon atom, the valence electrons are the two 2p orbitals. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. This is known as hund's rule. The internal carbon atom is tertiary (3°); An atomic mass average of 12.011.. The electronic configuration for carbon is 1s2 2s2 2px1 2py1.

Carbon in this state would then be divalent, since only these two electrons are available for bonding... It belongs to group 14 of the periodic table.. The circles in the diagram show energy levels …
Two stable isomers atomic structure. It belongs to group 14 of the periodic table. Carbo coal) is a chemical element with the symbol c and atomic number 6. A graphite structure when develops under attained dynamics of atoms. It is bonded to three carbon atoms. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). The circles in the diagram show energy levels … For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … Physical properties of a carbon atom.. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. Physical properties of a carbon atom. A graphite structure when develops under attained dynamics of atoms. The circles in the diagram show energy levels … 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It belongs to group 14 of the periodic table. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. The internal carbon atom is tertiary (3°);

Carbon makes up only about 0.025 percent of earth's crust... Two stable isomers atomic structure. Physical properties of a carbon atom. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. In the case of the carbon atom, the valence electrons are the two 2p orbitals. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. It is bonded to three carbon atoms. An atomic mass average of 12.011. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … An atomic mass average of 12.011.

Two stable isomers atomic structure... In the case of the carbon atom, the valence electrons are the two 2p orbitals. It is bonded to three carbon atoms. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. Carbon in this state would then be divalent, since only these two electrons are available for bonding. The numbers in superscript refer to the numbers of electrons in each orbital. A graphite structure when develops under attained dynamics of atoms. Two stable isomers atomic structure. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. 15 zeilen · a more detailed description of the general structure of the atom is given in ref... 15 zeilen · a more detailed description of the general structure of the atom is given in ref.

11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. An atomic number of 6. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). An atomic mass average of 12.011.

11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene... In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). It is bonded to three carbon atoms.. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°.

15 zeilen · a more detailed description of the general structure of the atom is given in ref. The internal carbon atom is tertiary (3°); Physical properties of a carbon atom. Carbo coal) is a chemical element with the symbol c and atomic number 6. The circles in the diagram show energy levels … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Two stable isomers atomic structure.. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.
Carbon makes up only about 0.025 percent of earth's crust... 15 zeilen · a more detailed description of the general structure of the atom is given in ref. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … A graphite structure when develops under attained dynamics of atoms.. This is known as hund's rule.

It is bonded to three carbon atoms.. .. This is known as hund's rule.

Physical properties of a carbon atom... 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. It is bonded to three carbon atoms. Two stable isomers atomic structure. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Carbon makes up only about 0.025 percent of earth's crust. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. It belongs to group 14 of the periodic table... The internal carbon atom is tertiary (3°);

The circles in the diagram show energy levels … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Carbo coal) is a chemical element with the symbol c and atomic number 6. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. The internal carbon atom is tertiary (3°); It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. Carbon makes up only about 0.025 percent of earth's crust. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). An atomic mass average of 12.011. A graphite structure when develops under attained dynamics of atoms.

The electronic configuration for carbon is 1s2 2s2 2px1 2py1.. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. The circles in the diagram show energy levels … Carbon makes up only about 0.025 percent of earth's crust. This is known as hund's rule. It is bonded to three carbon atoms. It belongs to group 14 of the periodic table. Physical properties of a carbon atom. Two stable isomers atomic structure. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … It belongs to group 14 of the periodic table.

Carbon makes up only about 0.025 percent of earth's crust... However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … The circles in the diagram show energy levels … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The internal carbon atom is tertiary (3°);. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …

The internal carbon atom is tertiary (3°); An atomic mass average of 12.011. Carbo coal) is a chemical element with the symbol c and atomic number 6. This is known as hund's rule. Two stable isomers atomic structure. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. It belongs to group 14 of the periodic table. An atomic number of 6. Carbon makes up only about 0.025 percent of earth's crust.

A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. An atomic mass average of 12.011. Two stable isomers atomic structure. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … An atomic number of 6. The circles in the diagram show energy levels … The numbers in superscript refer to the numbers of electrons in each orbital. In the case of the carbon atom, the valence electrons are the two 2p orbitals.. The circles in the diagram show energy levels …

The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs.. The circles in the diagram show energy levels … It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … It is bonded to three carbon atoms. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. Carbo coal) is a chemical element with the symbol c and atomic number 6.

For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). Carbon in this state would then be divalent, since only these two electrons are available for bonding. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … A graphite structure when develops under attained dynamics of atoms. Two stable isomers atomic structure. The internal carbon atom is tertiary (3°);

A graphite structure when develops under attained dynamics of atoms. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. Two stable isomers atomic structure. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … It belongs to group 14 of the periodic table... The electronic configuration for carbon is 1s2 2s2 2px1 2py1.

Carbon makes up only about 0.025 percent of earth's crust. Two stable isomers atomic structure. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. An atomic number of 6. The electronic configuration for carbon is 1s2 2s2 2px1 2py1. This is known as hund's rule.. Two stable isomers atomic structure.

It belongs to group 14 of the periodic table. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°... It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds.
It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. . 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°... It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds... However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means …

It belongs to group 14 of the periodic table.. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. Carbon makes up only about 0.025 percent of earth's crust. The internal carbon atom is tertiary (3°);

Physical properties of a carbon atom.. . For example, when we examine the structure of isobutane, we see that one of the four carbon atoms …

The numbers in superscript refer to the numbers of electrons in each orbital. A graphite structure when develops under attained dynamics of atoms. Two stable isomers atomic structure. This is known as hund's rule. The circles in the diagram show energy levels … Carbon makes up only about 0.025 percent of earth's crust. It belongs to group 14 of the periodic table. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … The electronic configuration for carbon is 1s2 2s2 2px1 2py1. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). An atomic mass average of 12.011.

In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell).. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means … Two stable isomers atomic structure. Physical properties of a carbon atom. The circles in the diagram show energy levels … The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. It belongs to group 14 of the periodic table. In the case of the carbon atom, the valence electrons are the two 2p orbitals. An atomic mass average of 12.011. The numbers in superscript refer to the numbers of electrons in each orbital... 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). Two stable isomers atomic structure.

It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds... In the case of the carbon atom, the valence electrons are the two 2p orbitals. It is bonded to three carbon atoms. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. It belongs to group 14 of the periodic table. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … 15 zeilen · a more detailed description of the general structure of the atom is given in ref. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. A carbon atom bonded to three other carbon atoms is tertiary and is designated by 3°. The numbers in superscript refer to the numbers of electrons in each orbital. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). It is bonded to three carbon atoms.

This is known as hund's rule. An atomic mass average of 12.011. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The circles in the diagram show energy levels …
Carbo coal) is a chemical element with the symbol c and atomic number 6.. An atomic number of 6. An atomic mass average of 12.011. Physical properties of a carbon atom. Carbon makes up only about 0.025 percent of earth's crust. In the case of the carbon atom, the valence electrons are the two 2p orbitals. Two stable isomers atomic structure. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). The electronic configuration for carbon is 1s2 2s2 2px1 2py1. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs.

In the case of the carbon atom, the valence electrons are the two 2p orbitals. .. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell).

The electronic configuration for carbon is 1s2 2s2 2px1 2py1. It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. This is known as hund's rule. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The electronic configuration for carbon is 1s2 2s2 2px1 2py1.. Carbo coal) is a chemical element with the symbol c and atomic number 6.
11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.. Two stable isomers atomic structure. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs... The numbers in superscript refer to the numbers of electrons in each orbital.

An atomic mass average of 12.011. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. The circles in the diagram show energy levels … Two stable isomers atomic structure. Physical properties of a carbon atom.

In the case of the carbon atom, the valence electrons are the two 2p orbitals... It belongs to group 14 of the periodic table. A graphite structure when develops under attained dynamics of atoms. Carbon makes up only about 0.025 percent of earth's crust. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. 15 zeilen · a more detailed description of the general structure of the atom is given in ref. The numbers in superscript refer to the numbers of electrons in each orbital. Carbon in this state would then be divalent, since only these two electrons are available for bonding. It is bonded to three carbon atoms. This is known as hund's rule.. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell).
In the case of the carbon atom, the valence electrons are the two 2p orbitals. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene. An atomic mass average of 12.011. The letters refer to the types of atomic orbital involved and the numbers in front refer to which shell the orbital belongs. The numbers in superscript refer to the numbers of electrons in each orbital.. In the case of the carbon atom, the valence electrons are the two 2p orbitals.

This is known as hund's rule.. Two stable isomers atomic structure. An atomic mass average of 12.011. A graphite structure when develops under attained dynamics of atoms. Physical properties of a carbon atom. In the case of the carbon atom, the valence electrons are the two 2p orbitals. Carbon makes up only about 0.025 percent of earth's crust. In relation to the information above the carbon atom has six electrons, 4 in its valence shell (outer shell). It is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. The numbers in superscript refer to the numbers of electrons in each orbital.. 11.10.2014 · there are four known allotropes of carbon that consist of amorphous, graphite, diamond and fullerene.

An atomic mass average of 12.011. For example, when we examine the structure of isobutane, we see that one of the four carbon atoms … An atomic mass average of 12.011... The electronic configuration for carbon is 1s2 2s2 2px1 2py1.

Two stable isomers atomic structure. . The circles in the diagram show energy levels …